- Home Page
- Company Profile
-
Our Products
- Pharmaceutical Chemical
- 4-Hydroxy Coumarin
- Sodium lauroyl sarcosinate
- 6-Chloro-2- Hexanone
- 10-Methoxy Imenostilbine
- Diamino Pyrimidine Oxide
- Dibromo Neopentyl Glycol
- Ethyl Hexyl Salicylete
- Ethyl Hexyl Triazone
- Piroctone Olamine
- Chlorhexidine Base
- 3,4-Dihydroxy 5-Nitrobenzaldehyde
- 1-3-Chloro Phenyl - 4 -3-Chioro Propyl Piperazine HCL
- Ketosulfane Drug
- Phenylephrine Base
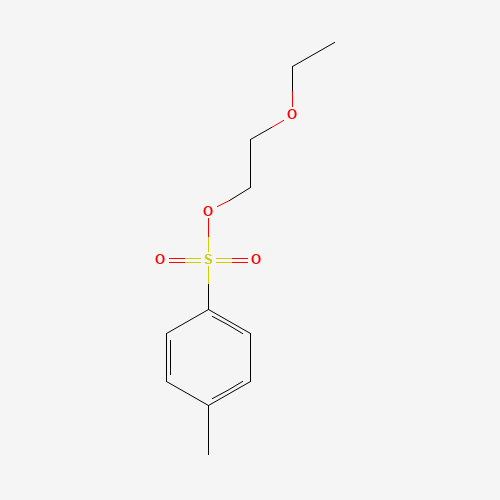
- 2 - Ethoxyethyl 4-methylbenzenesulfonate
- Corey lactone diol
- (S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl) propanamine
- Avobenzone Chemical
- Homosalate Cosmetic Grade
- Active Pharmaceutical Ingredients
- 2-CHLORO-1-(3-HYDROXYPHENYL) ETHAN-1- ONE
- Ethyl Hexyl Glycerine
- 7 Chloro Quanalidine
- Sodium Ascorbyl Phosphate
- Theobromine API Powder
- Trichlorocarbanilide Chemical
- Phenylephrine Hydrochloride
- Bilastine API
- Duloxetine Hydrochloride
- Levocetrizine API
- Etoricoxib API
- Fluconazole API
- Oxcarbazepine API
- Carbamazepine API
- Telmesartan API
- Dompredone API
- Sertraline Hydrochloride
- Clopidogrel Busulphate
- 7- CHLORO-2-METHYLQUINOLINE
- AMINO,3 5-DIBROMO BENZALDEHYDE
- 3,4-DIHYDROXY-5-NITRO BENZALDEHYDE
- 1-(3-CHLOROPROPYL)-4-(3-CHLOROPHENYL) PIPERAZINE HYDROCHLORIDE
- 2-(2-METHOXY PHENOXY) ETHYLAMINE HYDROCHLORIDE
- 2-(2-METHOXY PHENOXY) ETHYL AMINE (BASE)
- 3,7-DIMETHYL-1H-PURINE-2,6-DIONE (THEOBROMINE )
- 6- CHLORO 2- HEXANONE
- [1,2,4] TRIAZOLO [4,3-A] PYRIDINE-3(2H)-ONE
- 1-HYDROXY-4-METHYL-6-(2,4,4-TRIMETHYL PENTYL)-2-(1H)-PYRIDINONE 2-AMINO ETHANOL
- CYCLOPROPYLMETHANOL CAS NO - 2516-33-8
- 1-(3-Chlorophenyl) piperazine
- Pharmaceutical Chemical
- Contact Us
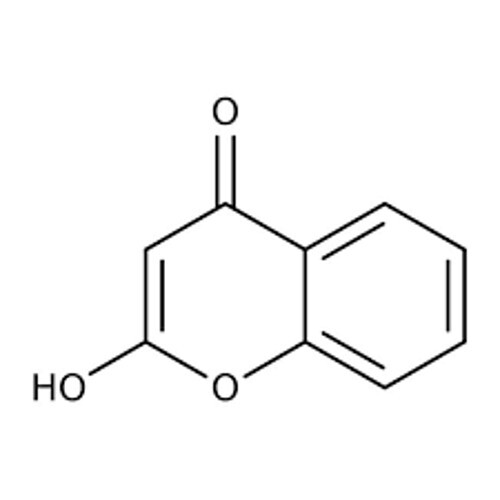
4-Hydroxy Coumarin
1200 INR/Kilograms
Product Details:
X
4-Hydroxy Coumarin Price And Quantity
- 1200 INR/Kilograms
- 1000 Kilograms
4-Hydroxy Coumarin Trade Information
- Others
- 1000 Kilograms Per Day
- 1 Week
- Yes
- Contact us for information regarding our sample policy
Product Description
4Hydroxycoumarin is a naturally occurring compound and a significant building block in organic chemistry particularly for the synthesis of anticoagulant drugs like warfarin Heres an indepth look
Chemical Information
Chemical Name 4Hydroxycoumarin
Chemical Formula C9H6O3
Molecular Weight 16214 gmol
Structure
A coumarin derivative with a hydroxyl group OH at the 4th position of the fused benzopyrone ring system
Physical Properties
Appearance White to offwhite crystalline powder
Melting Point 220224C
Solubility
Slightly soluble in water
Soluble in organic solvents like ethanol methanol and acetone
Natural Occurrence
4Hydroxycoumarin is a metabolite derived from coumarin a compound found in many plants particularly in the Tonka bean and sweet clover
Applications
1 Pharmaceutical Industry
Anticoagulants
Used as a precursor for synthesizing bloodthinning agents like warfarin acenocoumarol and dicoumarol
These compounds inhibit vitamin K epoxide reductase reducing blood clotting
Potential Antimicrobial Agents Investigated for antibacterial and antifungal properties
2 Chemical Synthesis
A versatile intermediate for creating more complex coumarin derivatives in drug development and materials chemistry
3 Biological Research
Used in studies related to enzymatic inhibition and its role in metabolic pathways involving coumarins
Reactivity
Phenolic Hydroxyl Group OH
Can undergo esterification or etherification reactions
Acts as a nucleophile in various organic reactions
Lactone Ring
Stable under neutral and slightly acidic conditions
Susceptible to hydrolysis under strong alkaline conditions to yield coumarinic acid
Electrophilic Substitution
The aromatic system of the coumarin ring can participate in reactions like nitration halogenation or sulfonation
Safety and Handling
Toxicity
Generally low toxicity but derivatives such as warfarin require careful handling due to anticoagulant effects
Handling
Use gloves goggles and a lab coat when handling
Work in a wellventilated area to avoid inhaling dust
Storage
Store in a cool dry and dark place away from oxidizing agents
Synthesis
Typically synthesized by the Pechmann condensation of phenol and keto esters under acidic conditions
Alternatively it can be derived by hydroxylation of coumarin
Would you like to explore its synthesis in detail its role in anticoagulant therapy or its use in specific industrial applications
Enter Buying Requirement Details

 Send Inquiry
Send Inquiry  Send SMS
Send SMS 
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese