- Home Page
- Company Profile
-
Our Products
- Active Pharmaceutical Ingredients
- CYCLOPROPYLMETHANOL CAS NO - 2516-33-8
- 7 Chloro Quanalidine
- 2-CHLORO-1-(3-HYDROXYPHENYL) ETHAN-1- ONE
- 3,4-DIHYDROXY-5-NITRO BENZALDEHYDE
- Oxcarbazepine API
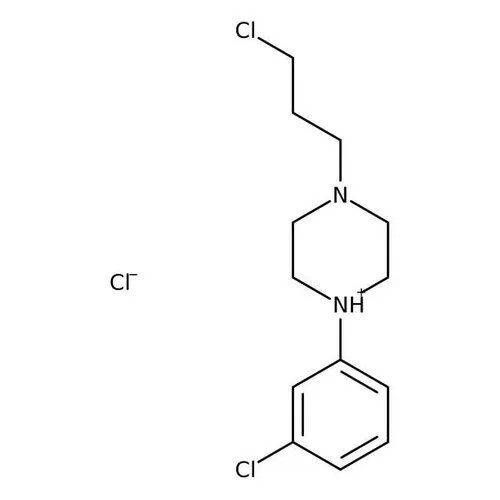
- 1-(3-CHLOROPROPYL)-4-(3-CHLOROPHENYL) PIPERAZINE HYDROCHLORIDE
- Duloxetine Hydrochloride
- Trichlorocarbanilide Chemical
- 3,7-DIMETHYL-1H-PURINE-2,6-DIONE (THEOBROMINE )
- AMINO,3 5-DIBROMO BENZALDEHYDE
- 2-(2-METHOXY PHENOXY) ETHYLAMINE HYDROCHLORIDE
- Sertraline Hydrochloride
- Levocetrizine API
- Dompredone API
- Sodium Ascorbyl Phosphate
- Carbamazepine API
- 1-HYDROXY-4-METHYL-6-(2,4,4-TRIMETHYL PENTYL)-2-(1H)-PYRIDINONE 2-AMINO ETHANOL
- Phenylephrine Hydrochloride
- Bilastine API
- Ethyl Hexyl Glycerine
- Telmesartan API
- Theobromine API Powder
- Etoricoxib API
- Fluconazole API
- [1,2,4] TRIAZOLO [4,3-A] PYRIDINE-3(2H)-ONE
- Clopidogrel Busulphate
- 2-(2-METHOXY PHENOXY) ETHYL AMINE (BASE)
- 6- CHLORO 2- HEXANONE
- Pharmaceutical Intermediate
- 1-(3-Chlorophenyl) piperazine
- Corey lactone diol
- 6-Chloro-2- Hexanone
- Piroctone Olamine
- Phenylephrine Base
- Ethyl Hexyl Salicylete
- 4-Hydroxy Coumarin
- Diamino Pyrimidine Oxide
- Avobenzone Chemical
- Dibromo Neopentyl Glycol
- 3,4-Dihydroxy 5-Nitrobenzaldehyde
- Chlorhexidine Base
- Ethyl Hexyl Triazone
- (S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl) propanamine
- Ketosulfane Drug
- Homosalate Cosmetic Grade
- 1-3-Chloro Phenyl - 4 -3-Chioro Propyl Piperazine HCL
- 10-Methoxy Imenostilbine
- 2-Amino-3,5-Dibromo Benzaldehyde
- 2 - Ethoxyethyl 4-methylbenzenesulfonate
- Active Pharmaceutical Ingredients
- Contact Us
CYCLOPROPYLMETHANOL CAS NO - 2516-33-8
1892 INR/Kilograms
Product Details:
X
CYCLOPROPYLMETHANOL CAS NO - 2516-33-8 Price And Quantity
- 1000 Kilograms
- 1892 INR/Kilograms
CYCLOPROPYLMETHANOL CAS NO - 2516-33-8 Trade Information
- Others
- 1000 Kilograms Per Day
- 1 Week
- Yes
- Contact us for information regarding our sample policy
Product Description
Cyclopropylmethanol is an organic compound with a cyclopropyl group attached to a methanol structure Heres a detailed description of the compound
Chemical Information
Chemical Name Cyclopropylmethanol
Chemical Formula C4H6O
Molecular Weight 7009 gmol
IUPAC Name Cyclopropylmethanol
Structure
The compound consists of a cyclopropyl group a threemembered ring with two single bonds and one strained bond attached to a methanol group a CH2OH group which contains a hydroxymethyl group
It is a simple alcohol with a unique threemembered ring structure that is known for its reactivity due to the strain in the cyclopropyl ring
Physical Properties
Appearance A colorless liquid typically transparent in appearance
Boiling Point Approximately 160162C
Melting Point 30C approximately
Density 098 gcm at 20C
Solubility
Soluble in water as it contains the hydroxymethyl CH2OH group
Soluble in organic solvents like ethanol diethyl ether and chloroform
Reactivity and Chemistry
Cyclopropyl Group Reactivity
The cyclopropyl group is highly strained which makes it reactive and prone to undergoing ringopening reactions in the presence of nucleophiles or under heat
It can undergo nucleophilic substitution reactions and is often used as a reactive intermediate in organic synthesis The strain in the ring makes it susceptible to ringopening reactions especially under basic or acidic conditions
Hydroxymethyl Group CH2OH
The hydroxymethyl group makes the molecule an alcohol and capable of participating in hydrogen bonding interactions
It can also undergo oxidation to form formaldehyde if the hydroxymethyl group is oxidized or further functionalization in synthetic reactions
Reactivity in Organic Synthesis
Cyclopropylmethanol is useful in organic chemistry for ringopening reactions and as a precursor to other cyclopropylcontaining compounds
It may also serve as a building block for the synthesis of more complex molecules or as an intermediate in the preparation of cyclic compounds
Applications
1 Organic Synthesis
Cyclopropylmethanol is often used as an intermediate in organic synthesis The strained cyclopropyl ring can be opened under various conditions leading to the formation of new carboncarbon bonds
It is useful in the synthesis of pharmaceuticals agrochemicals and fine chemicals
2 Pharmacological Interest
The cyclopropyl group is known to impart specific steric and electronic effects which may influence the biological activity of compounds that contain it
Cyclopropylmethanol itself may not have significant bioactivity but could serve as a precursor to biologically active molecules or compounds used in drug design
3 Industrial Applications
In the industrial context cyclopropylmethanol may be involved in the production of flavor compounds fragrances or other specialized chemicals due to its functional group and structure
Safety and Handling
Toxicity
Cyclopropylmethanol is generally considered to be of low toxicity but like most chemicals it should be handled with care
It may cause irritation to the skin eyes and respiratory tract if inhaled or if it comes into contact with skin
Handling and Storage
Cyclopropylmethanol should be stored in a cool dry and wellventilated area away from sources of heat or flame
Proper personal protective equipment PPE such as gloves and goggles should be used during handling to prevent direct contact
Synthesis
Cyclopropylmethanol can be synthesized via several methods including
1 Cyclization of Hydroxymethyl Groups
It can be prepared from a suitable precursor with a hydroxymethyl group using a cyclization reaction under the right conditions
2 Reduction of Cyclopropylcarbinyl Alcohols
Cyclopropylmethanol can also be obtained through the reduction of cyclopropylcarbinyl alcohols in a controlled reaction potentially using reagents like lithium aluminum hydride LiAlH
3 RingOpening of Cyclopropyl Compounds
The compound can be synthesized by the ringopening of more complex cyclopropyl compounds often in the presence of a nucleophile such as water or alcohol
Conclusion
Cyclopropylmethanol is a versatile compound with a unique structure that combines a highly reactive cyclopropyl group with a hydroxymethyl group Its chemical reactivity particularly the ability to undergo ringopening reactions makes it a useful intermediate in organic synthesis It can also be a building block for the design of more complex molecules in pharmaceuticals and agrochemicals
Would you like further exploration into its synthetic methods applications or reactivity in specific reactions
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email


 Send Inquiry
Send Inquiry  Send SMS
Send SMS  Call Me Free
Call Me Free 
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese