- Home Page
- Company Profile
-
Our Products
- Active Pharmaceutical Ingredients
- CYCLOPROPYLMETHANOL CAS NO - 2516-33-8
- 7 Chloro Quanalidine
- 2-CHLORO-1-(3-HYDROXYPHENYL) ETHAN-1- ONE
- 3,4-DIHYDROXY-5-NITRO BENZALDEHYDE
- Oxcarbazepine API
- 1-(3-CHLOROPROPYL)-4-(3-CHLOROPHENYL) PIPERAZINE HYDROCHLORIDE
- Duloxetine Hydrochloride
- Trichlorocarbanilide Chemical
- 3,7-DIMETHYL-1H-PURINE-2,6-DIONE (THEOBROMINE )
- AMINO,3 5-DIBROMO BENZALDEHYDE
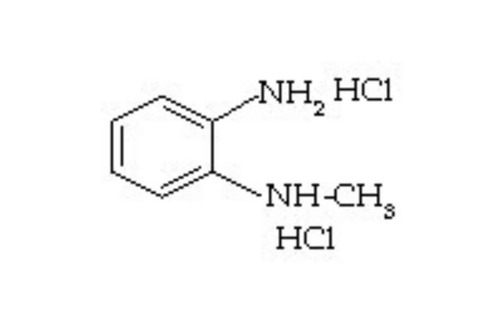
- 2-(2-METHOXY PHENOXY) ETHYLAMINE HYDROCHLORIDE
- Sertraline Hydrochloride
- Levocetrizine API
- Dompredone API
- Sodium Ascorbyl Phosphate
- Carbamazepine API
- 1-HYDROXY-4-METHYL-6-(2,4,4-TRIMETHYL PENTYL)-2-(1H)-PYRIDINONE 2-AMINO ETHANOL
- Phenylephrine Hydrochloride
- Bilastine API
- Ethyl Hexyl Glycerine
- Telmesartan API
- Theobromine API Powder
- Etoricoxib API
- Fluconazole API
- [1,2,4] TRIAZOLO [4,3-A] PYRIDINE-3(2H)-ONE
- Clopidogrel Busulphate
- 2-(2-METHOXY PHENOXY) ETHYL AMINE (BASE)
- 6- CHLORO 2- HEXANONE
- Pharmaceutical Intermediate
- 1-(3-Chlorophenyl) piperazine
- Corey lactone diol
- 6-Chloro-2- Hexanone
- Piroctone Olamine
- Phenylephrine Base
- Ethyl Hexyl Salicylete
- 4-Hydroxy Coumarin
- Diamino Pyrimidine Oxide
- Avobenzone Chemical
- Dibromo Neopentyl Glycol
- 3,4-Dihydroxy 5-Nitrobenzaldehyde
- Chlorhexidine Base
- Ethyl Hexyl Triazone
- (S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl) propanamine
- Ketosulfane Drug
- Homosalate Cosmetic Grade
- 1-3-Chloro Phenyl - 4 -3-Chioro Propyl Piperazine HCL
- 10-Methoxy Imenostilbine
- 2-Amino-3,5-Dibromo Benzaldehyde
- 2 - Ethoxyethyl 4-methylbenzenesulfonate
- Active Pharmaceutical Ingredients
- Contact Us
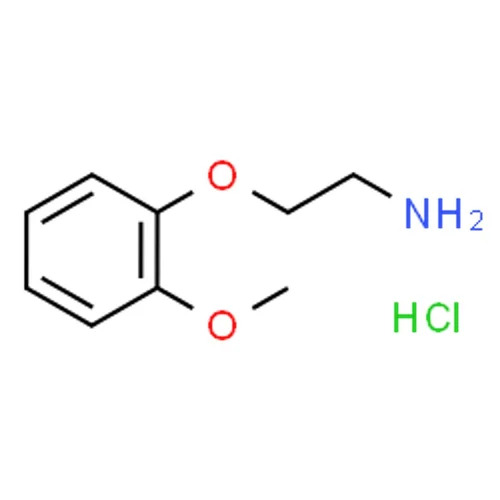
2-(2-METHOXY PHENOXY) ETHYL AMINE (BASE)
6900 INR/Kilograms
Product Details:
X
2-(2-METHOXY PHENOXY) ETHYL AMINE (BASE) Price And Quantity
- 6900 INR/Kilograms
- 1000 Kilograms
2-(2-METHOXY PHENOXY) ETHYL AMINE (BASE) Trade Information
- Others
- 1000 Kilograms Per Week
- 1 Week
- Yes
- Contact us for information regarding our sample policy
Product Description
22Methoxyphenoxyethylamine Base is the freebase form of the compound differing from the hydrochloride salt by the absence of the protonated amine and chloride counterion Below is a detailed description
Chemical Information
Chemical Name 22Methoxyphenoxyethylamine Base
Chemical Formula C9H13NO2
Molecular Weight 16721 gmol
Structure
Phenoxy Group A benzene ring substituted with a methoxy group OCH3 at the 2nd position and linked via an ether bond O to the ethyl chain
Amino Group NH2 Attached to the terminal carbon of the ethyl chain
Physical Properties
Appearance A clear liquid or paleyellow solid depending on temperature and purity
Melting Point Usually lower than the hydrochloride salt due to the absence of ionic interactions
Boiling Point Approximately 260280C
Solubility
Soluble in organic solvents like ethanol methanol or acetone
Slightly soluble in water compared to its hydrochloride salt
Applications
1 Pharmaceutical Intermediate
Used in the synthesis of therapeutic agents such as betablockers and adrenergic receptor modulators
Key precursor in the production of antihypertensive and antiarrhythmic drugs
2 Organic Synthesis
Versatile intermediate for creating more complex molecules in research and chemical industries
Phenoxy and amine groups make it amenable to further functionalization
3 Material Science
Used as a building block for creating polymers or advanced materials with specific properties
Safety and Handling
Hazards
Flammable keep away from heat and open flames
May cause irritation to the skin eyes and respiratory tract Avoid prolonged exposure
Handling
Use standard laboratory precautions gloves goggles and lab coat
Ensure work is conducted in a fume hood or wellventilated area
Storage
Store in a tightly sealed container in a cool dry and ventilated area
Protect from exposure to light and moisture
Reactivity
Functional Groups
Amino Group NH2 Highly reactive can participate in acylation alkylation or Schiff base formation
Ether Bond O Chemically stable under neutral conditions but may degrade under strong acidic or basic environments
Methoxy Group OCH3 Electrondonating enhances reactivity of the benzene ring in electrophilic substitution reactions
Would you like additional details on its synthesis potential reactions or applications in drug development
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email



 Send Inquiry
Send Inquiry  Send SMS
Send SMS  Call Me Free
Call Me Free 
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese