- Home Page
- Company Profile
-
Our Products
- Active Pharmaceutical Ingredients
- CYCLOPROPYLMETHANOL CAS NO - 2516-33-8
- 7 Chloro Quanalidine
- 2-CHLORO-1-(3-HYDROXYPHENYL) ETHAN-1- ONE
- 3,4-DIHYDROXY-5-NITRO BENZALDEHYDE
- Oxcarbazepine API
- 1-(3-CHLOROPROPYL)-4-(3-CHLOROPHENYL) PIPERAZINE HYDROCHLORIDE
- Duloxetine Hydrochloride
- Trichlorocarbanilide Chemical
- 3,7-DIMETHYL-1H-PURINE-2,6-DIONE (THEOBROMINE )
- AMINO,3 5-DIBROMO BENZALDEHYDE
- 2-(2-METHOXY PHENOXY) ETHYLAMINE HYDROCHLORIDE
- Sertraline Hydrochloride
- Levocetrizine API
- Dompredone API
- Sodium Ascorbyl Phosphate
- Carbamazepine API
- 1-HYDROXY-4-METHYL-6-(2,4,4-TRIMETHYL PENTYL)-2-(1H)-PYRIDINONE 2-AMINO ETHANOL
- Phenylephrine Hydrochloride
- Bilastine API
- Ethyl Hexyl Glycerine
- Telmesartan API
- Theobromine API Powder
- Etoricoxib API
- Fluconazole API
- [1,2,4] TRIAZOLO [4,3-A] PYRIDINE-3(2H)-ONE
- Clopidogrel Busulphate
- 2-(2-METHOXY PHENOXY) ETHYL AMINE (BASE)
- 6- CHLORO 2- HEXANONE
- Pharmaceutical Intermediate
- 1-(3-Chlorophenyl) piperazine
- Corey lactone diol
- 6-Chloro-2- Hexanone
- Piroctone Olamine
- Phenylephrine Base
- Ethyl Hexyl Salicylete
- 4-Hydroxy Coumarin
- Diamino Pyrimidine Oxide
- Avobenzone Chemical
- Dibromo Neopentyl Glycol
- 3,4-Dihydroxy 5-Nitrobenzaldehyde
- Chlorhexidine Base
- Ethyl Hexyl Triazone
- (S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl) propanamine
- Ketosulfane Drug
- Homosalate Cosmetic Grade
- 1-3-Chloro Phenyl - 4 -3-Chioro Propyl Piperazine HCL
- 10-Methoxy Imenostilbine
- 2-Amino-3,5-Dibromo Benzaldehyde
- 2 - Ethoxyethyl 4-methylbenzenesulfonate
- Active Pharmaceutical Ingredients
- Contact Us
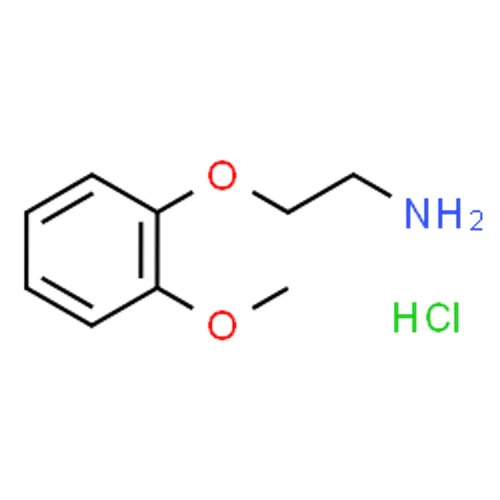
2-(2-METHOXY PHENOXY) ETHYLAMINE HYDROCHLORIDE
6960 INR/Kilograms
Product Details:
- Type Other
- Click to View more
X
2-(2-METHOXY PHENOXY) ETHYLAMINE HYDROCHLORIDE Price And Quantity
- 1000 Kilograms
- 6960 INR/Kilograms
2-(2-METHOXY PHENOXY) ETHYLAMINE HYDROCHLORIDE Product Specifications
- Other
2-(2-METHOXY PHENOXY) ETHYLAMINE HYDROCHLORIDE Trade Information
- Others
- 1000 Kilograms Per Day
- 1 Week
- Yes
- Contact us for information regarding our sample policy
Product Description
22Methoxyphenoxyethylamine hydrochloride is an organic compound widely used as an intermediate in pharmaceuticals and fine chemicals Below is a detailed description
Chemical Information
Chemical Name 22Methoxyphenoxyethylamine hydrochloride
Chemical Formula C9H13NO2 HCl
Molecular Weight 20367 gmol
Structure
Phenoxy Group A benzene ring substituted with a methoxy group OCH3 at the 2nd position and linked via an ether bond O to the ethyl chain
Amino Group NH2 Attached to the terminal carbon of the ethyl chain
Hydrochloride Salt Protonated amine group neutralized by a chloride ion
Physical Properties
Appearance A crystalline solid typically white or offwhite
Melting Point Around 160170C may vary based on purity
Solubility
Highly soluble in water due to the hydrochloride salt form
Soluble in polar organic solvents such as methanol or ethanol
Applications
1 Pharmaceutical Industry
Used as an intermediate in synthesizing various drugs including betablockers and antihypertensive agents
Precursor in developing compounds with adrenergic receptor activity
2 Chemical Synthesis
A versatile building block in organic chemistry for creating more complex molecules
The phenoxy and amine functionalities allow for further derivatization
3 Research and Development
Often employed in medicinal chemistry studies to design biologically active molecules
Safety and Handling
Hazards
May cause irritation to skin eyes and respiratory tract
Avoid inhalation and direct contact
Handling
Use appropriate personal protective equipment PPE including gloves safety goggles and a lab coat
Work in a wellventilated area or under a fume hood
Storage
Store in a cool dry place away from moisture and strong oxidizing agents
Reactivity
Functional Groups
Amino Group NH2 Can undergo acylation alkylation or Schiff base formation
Ether Bond O Stable under most conditions but susceptible to cleavage under strong acidic or basic environments
Methoxy Group OCH3 Increases the electron density of the aromatic ring enhancing reactivity toward electrophilic substitution
Let me know if you require more specific details such as synthesis routes spectral characterization NMR IR MS or potential derivatives
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email


 Send Inquiry
Send Inquiry  Send SMS
Send SMS  Call Me Free
Call Me Free 
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese